Canada Gazette, Part I, Volume 150, Number 49: Children's Jewellery Regulations
December 3, 2016
Statutory authority
Canada Consumer Product Safety Act
Sponsoring department
Department of Health
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Executive summary
Issues: The adverse health effects of lead on young children have been documented in numerous studies. Children absorb a much greater percentage of ingested lead than adults do, and their developing organs and body systems are more susceptible to the toxic effects of lead. Cadmium is also a very toxic metal. High levels of cadmium have been found in children's jewellery on the Canadian marketplace within the last five years.
Young children are much more likely to be exposed to lead and cadmium in children's jewellery because of their natural habit of mouthing objects. Numerous incidents of children mouthing or swallowing jewellery have been recorded.
Description: This proposal would amend the Children's Jewellery Regulations under the Canada Consumer Product Safety Act to (1) add a 130 milligram per kilogram (mg/kg) total cadmium limit for children's jewellery items small enough to be swallowed by a child; and (2) replace the current 600 mg/kg total lead limit and 90 mg/kg migratable lead limit with a single 90 mg/kg total lead limit for all children's jewellery items.
Cost-benefit statement: A cost-benefit analysis for this regulatory proposal was completed in March 2014. Qualitative benefits identified included avoidance of fatalities, illnesses and consequent medical costs, loss of lifetime earnings, and costs for special education, justice and corrections. Total industry costs were estimated at $4.5 million ($4.6 million in 2016 dollars) over 20 years when discounted at 7%. A break-even analysis indicated that benefits would outweigh costs if one fatality was avoided within approximately six years of adopting the proposed limits.
“One-for-One” Rule and small business lens: The “One-for-One” Rule does not apply, since the Regulations do not impose any administrative costs on industry. The small business lens also does not apply because the estimated nationwide cost impact is less than $1 million per year.
Domestic and international coordination and cooperation: The lead content limit of the proposed amendments is consistent with the international health and safety objective of reducing the intentional use of lead as much as possible, although the actual lead limits may differ slightly from one jurisdiction to another. The regulatory proposal also ensures that the lead limits are consistent across the Canadian regulatory regime.
The proposed cadmium limit is comparable to the European Union (EU) limit of 100 mg/kg total cadmium for costume jewellery. The United States does not have federal cadmium limits for children's jewellery.
Background
Lead is a heavy metal that is very toxic, (see footnote 1), (see footnote 2) especially to children. Young children are also more likely to be exposed to lead because of their natural habit of mouthing objects. Lead has historically been used in children's jewellery because it is inexpensive and easily molded. A number of lead poisoning cases in children, including one fatality in the United States, have been associated with the chewing, sucking or swallowing of jewellery with high lead content.
The Children's Jewellery Regulations (CJR), which came into force in 2005, currently restrict the use of lead in jewellery items intended for children under the age of 15 years to 600 mg/kg total lead and 90 mg/kg migratable (see footnote 3) lead.
Cadmium is also a highly toxic heavy metal. Unlike lead, which has a sweet taste, cadmium tastes very bitter and it is unlikely that children would regularly chew or suck cadmium-containing jewellery. However, Health Canada scientists have concluded that there is potential for serious health effects in children who swallow jewellery items made with cadmium. Ingested cadmium has been associated with adverse effects on the kidneys, liver, heart, blood system, nervous system, reproductive system and immune system.
There is no risk to human health from simply wearing children's jewellery containing high levels of lead and/or cadmium; however, Health Canada has reason to believe that the unintentional ingestion of jewellery by children occurs frequently. The United States Consumer Product Safety Commission (U.S. CPSC) reported in 2006 that from 2000 to 2005, there were more than 300 000 emergency room visits in the United States by children 18 years of age or below because of foreign object ingestion. It was estimated that 80% of these children were below 7 years of age and that approximately 20 000 of the swallowed objects were jewellery items.
In 2009–2010, Health Canada carried out a survey of children's jewellery products to determine the level of marketplace compliance with the lead content limits of the CJR. Because of concerns that manufacturers may have been increasingly substituting scrap cadmium for lead in children's jewellery, all 103 survey samples were also tested for cadmium. Very high cadmium levels, up to 93% (930 000 mg/kg), were found in some of the samples. Health Canada issued a consumer advisory on lead and cadmium in children's jewellery on January 10, 2010. (see footnote 4)
Children's jewellery items with high cadmium content were also found at the same time on the American market. In 2010, the U.S. CPSC announced a number of recalls of children's jewellery items because of high levels of cadmium.
Issues
Children who chew, suck or swallow children's jewellery containing lead and/or cadmium are at serious risk of adverse, even fatal, health effects. It is difficult for parents and caregivers to protect children against lead or cadmium in children's jewellery because it is not possible to accurately determine whether an item contains lead or cadmium without tests, which cannot feasibly be carried out by the general public.
Lead
Since 2005, Health Canada has reduced existing total lead content limits for products such as consumer paints and other surface coating materials, toys for children under three years of age, and applied surface coatings on children's articles to the more stringent level of 90 mg/kg. However, for children's jewellery, the total lead content limit remains at 600 mg/kg. This limit is not consistent with the 90 mg/kg total lead limit in effect or proposed under the Canada Consumer Product Safety Act (CCPSA) for other products posing a similar risk.
Calculations by Health Canada based on the World Health Organization provisional tolerable daily intake of 3.75 micrograms of lead per kilogram of body weight (established in 1987 and reconfirmed in 2002) showed that a 600 mg/kg total lead limit could expose children to harmful amounts of lead and that a 90 mg/kg total lead limit would provide adequate protection to children against lead exposure through affected products.
Since no “safe” level of lead in the blood has been identified by scientists, the potential for exposure to lead through consumer products such as children's jewellery should be reduced to the maximum extent possible.
Cadmium
Historically, the use of cadmium in consumer products has been limited to a few special applications. However, following the introduction of stringent lead limits, suppliers apparently began substituting cadmium for lead in children's jewellery.
As a result of the discovery of children's jewellery items with very high cadmium content on the Canadian marketplace during Health Canada's 2009–2010 survey, the Minister of Health called on industry, in October 2010, to voluntarily stop the production, import, and sale of children's jewellery items containing cadmium. The Minister also indicated that if there were no improvement, regulatory measures might be put in place. The results of follow-up marketplace surveys by Health Canada in 2011, 2012 and 2013 showed that children's jewellery items containing high cadmium levels were still available on the Canadian marketplace.
Health Canada toxicologists carried out a risk assessment (see footnote 5) in 2011 to identify a cadmium limit for children's jewellery that would sufficiently protect children against cadmium exposure if they swallowed a children's jewellery item. A limit of 130 mg/kg total cadmium was identified as being sufficiently protective of children under the worst-case exposure scenario, in which a child may swallow a children's jewellery item that then becomes lodged in the stomach over an extended period.
Currently, there are no regulatory limits in Canada for cadmium in children's jewellery. Health Canada can take action against children's jewellery containing cadmium only by use of the general prohibitions (GPs) in the CCPSA, which prohibit the manufacture, import, advertisement or sale of consumer products that are a danger to human health or safety.
Objectives
The objective of the proposed amendments to the CJR is to help protect children against serious risks to health from chewing, sucking or swallowing children's jewellery containing lead or cadmium by introducing a limit on total cadmium content and further restricting total lead levels in children's jewellery products to levels more protective than current requirements.
Description
The proposed amendments to the CJR would
- (1) replace the current 600 mg/kg total lead limit and 90 mg/kg migratable lead limit with a 90 mg/kg total lead limit for all children's jewellery items; and
- (2) add a limit of 130 mg/kg total cadmium for children's jewellery items that are small enough to be totally enclosed in a small parts cylinder (the small parts cylinder is an internationally recognized gauge used to determine whether an item can be swallowed by a child).
Regulatory and non-regulatory options considered
Option 1: Status quo
Cadmium — Health Canada could continue to take action against cadmium in children's jewellery by invoking the GP in the CCPSA on the manufacture, import, advertisement or sale of consumer products that are a danger to the health or safety of Canadians. Although this approach has been taken with other consumer products/hazards, and has successfully demonstrated a key aspect of the modern tools and authorities afforded by the CCPSA, invoking the GP requires the extra burden of a risk assessment to support compliance actions. Establishing a fixed total cadmium limit in regulations would allow Health Canada to take swifter action on non-compliant products and provides greater clarity for industry regarding the acceptable limit.
Lead — The current limits of 600 mg/kg total lead and 90 mg/kg migratable lead do not reflect the fact that no threshold of safety for lead exposure has been identified and that harmful health effects, especially to children, may occur at very low levels of lead exposure. They are also inconsistent with current lead limits under the CCPSA for products posing a similar exposure risk to children.
The existence of a migratable limit in addition to a total lead limit also creates potential for additional industry costs, since migratable lead testing is required if the total lead content is between 90 mg/kg and 600 mg/kg.
This option was rejected because it would result in the continuation of an unnecessary cadmium and lead exposure risk from children's jewellery, would provide insufficient clarity on acceptable cadmium limits, and is not consistent with lead limits currently in effect or proposed for other products that pose a similar lead exposure risk.
Option 2: Voluntary industry standards for cadmium and retention of the current lead limits in the CJR
Despite the Minister of Health's October 2010 challenge to industry to voluntarily discontinue the use of cadmium in children's jewellery, follow-up surveys carried out by Health Canada in 2011, 2012, and 2013 determined that children's jewellery with high cadmium levels were still being sold in Canada. These results showed that the request for voluntary action had been ineffective in removing children's jewellery with dangerous levels of cadmium from the marketplace.
Similarly, in 1999 and again in 2000, Health Canada requested industry to voluntarily refrain from selling children's jewellery made with lead. Follow-up surveys in 1999 and 2001 showed that the voluntary request had been ineffective and items with high lead content were still being marketed in Canada.
Voluntary standards are generally effective only when endorsed and enforced by industry. The children's jewellery industry in Canada is fragmented and constantly changing, with many distribution channels and retail outlets. Almost all the manufacturers and suppliers marketing children's jewellery in Canada are based in other countries, mainly in Asia. As a result, it would be difficult to obtain commitment or effective enforcement from industry for a voluntary Canadian standard.
This option was rejected because it would mean retention of the current lead content limits under the CJR, which are inconsistent with current limits for other products with similar lead exposure risks and with the current lack of an identified “safe” lower limit for lead exposure. It would also create an incompatibility between the way lead exposure risks and cadmium exposure risks from children's jewellery are managed since the risk of exposure to lead in children's jewellery is currently managed through specific regulatory limits on lead content.
Option 3: Addition of option of the cadmium requirements of ASTM F2923-14 Standard Specification for Consumer Product Safety for Children's Jewelry and retention of the current lead limits in the CJR
The United States does not have a federal regulatory limit for cadmium in children's jewellery. In 2011, the U.S. ASTM standards organization published a voluntary standard, F2923-11 Standard Specification for Consumer Product Safety for Children's Jewelry, which includes cadmium limits. It does not have any total cadmium limit but requires migratable cadmium testing if total cadmium is greater than 300 mg/kg. The migratable cadmium limits vary depending on the material being tested and the size of the jewellery.
Scientific data on the toxicity of ingested cadmium to humans is very limited. Health Canada has conducted studies on the migration of cadmium from children's jewellery over extended time periods, and the results indicate that limits based on migratable content alone do not offer sufficient protection to children. Migration tests for periods of 24 hours or less (as defined by the ASTM standard) do not reflect the most likely exposure scenario in which a child swallows a piece of jewellery that subsequently becomes lodged in the child's stomach, continually releasing cadmium.
Migration testing by Health Canada also found that there was no consistent relationship between the total cadmium content of a jewellery sample and the amount of cadmium that might be released from it in the simulated environment of the stomach over an extended period of time. Since standardized migration testing does not accurately predict the amount of cadmium that might be leached out of a swallowed children's jewellery item, use of a total cadmium limit is considered the most health-protective approach.
It should be noted that products compliant with the preferred option (option 4) would also be compliant with this option. Compliance with the 130 mg/kg total cadmium limit would also ensure compliance with the ASTM F2923-14 standard, without the need for industry to carry out costly tests for migratable cadmium.
Under this option, the current lead limits under the CJR would be retained. This option was rejected because these limits are inconsistent with current or proposed lead limits for other products posing similar lead exposure risks and with the fact that no threshold of safety for lead exposure has been identified.
Option 4: Adoption of the 130 mg/kg total cadmium limit for items small enough to be swallowed by a child and replacement of the current 600 mg/kg total and 90 mg/kg migratable lead limits for children's jewellery with a single limit of 90 mg/kg total lead in the CJR
This is the option represented by the current regulatory proposal and was chosen for the following reasons:
- The proposed limit of 130 mg/kg total cadmium includes a margin of safety that helps to provide protection for children.
- Specific regulatory requirements for cadmium in children's jewellery would provide certainty and predictability to industry and facilitate compliance and enforcement action when required. They would allow Health Canada to take immediate compliance and enforcement action without having to demonstrate on a case-by-case basis that a specific product posed a “danger to human health and safety”;
- The proposed cadmium requirements focus on items small enough to be swallowed because the main cadmium exposure risk is from swallowed items. Because cadmium tastes very bitter, children are not likely to suck or chew on items made with cadmium.
- Introduction of a specific cadmium limit for children's jewellery in regulations is consistent with the way risks from lead in children's jewellery are currently managed.
- Replacement of the 600 mg/kg total lead limit and 90 mg/kg migratable lead limit with a 90 mg/kg total lead limit would align lead limits for children's jewellery with limits currently in effect or proposed under the CCPSA for other products posing a similar lead exposure risk.
- Since total cadmium and lead tests are less costly than migratable tests, introduction of total lead and cadmium limits would reduce industry costs for testing children's jewellery.
Benefits and costs
A cost-benefit analysis of this proposal was carried out between November 2013 and March 2014. A survey questionnaire was distributed to 27 industry stakeholders, including one Canadian manufacturer, 12 wholesalers/retailers, 10 retailers, 2 industry associations, including an association representing small business, one regulatory agency in the United States, and a supplier of metaltesting equipment. Responses were received from 8 companies and one U.S.-based industry association.
Seven of the companies indicated that they did not expect any significant costs from the amendments. The remaining company indicated that it would incur significant compliance costs. Industry respondents in general expressed the view that regulatory requirements for children's jewellery should be harmonized with those of the United States.
The study estimated that the market for children's jewellery in Canada was in the range of $35 million per year at retail.
Table 1: Summary of economic impacts of proposed regulatory amendments
| Base Year | Total (PV) (see footnote 6) | Annualized Average | ||
|---|---|---|---|---|
| Negative impacts | By stakeholder | 2016 | ||
| Compliance costs | Industry | 4.6 | 0.44 | |
| Qualitative impacts | ||||
Consumer (negative)
|
||||
Costs to industry
The 2013–14 cost-benefit analysis identified estimated industry costs in undiscounted 2016 Canadian dollars over 20 years. Discounted at 7%, the total costs would be $4.6 million over 20 years. Industry costs included an average of $0.25 per non-compliant piece to procure compliant metals and metal mixes, and an average of $0.25 per piece for additional testing and certification costs, assumed to apply to all pieces of children's jewellery sold in Canada. The costs were assumed to be similar for all companies.
These costs would initially be borne by (predominantly foreign) children's jewellery manufacturers and are expected to be passed through the supply chain to consumers.
Costs to consumers
The cost-benefit analysis assumed that nearly all costs to industry would ultimately be borne by Canadian buyers of children's jewellery and that the average price increase per piece of jewellery would be in the range of $0.10.
The proposed amendments may also potentially result in reduced consumer choice, since some companies chose to stop marketing children's jewellery after lead content limits were introduced in 2005. It is likely that other companies may choose to stop marketing children's jewellery rather than incur additional costs to meet the new limits.
Costs to Government
Health Canada would be responsible for the implementation of the proposed amendments and for related compliance and enforcement activities. These activities would be a part of Health Canada's regular compliance and enforcement program for consumer products. The Department would not incur any incremental capital or operating costs due to the proposed amendments.
Benefits to Canadians
The 2014 cost-benefit study for this regulatory proposal identified a number of major gaps in the data necessary to estimate the benefits of this proposal. Data gaps included
- the share of children's jewellery on store shelves that has a total lead concentration between 90 and 600 mg/kg and a cadmium concentration above 130 mg/kg;
- the probability that a fatality would occur arising from a piece of children's jewellery with a total lead concentration between 90 and 600 mg/kg and a cadmium concentration above 130 mg/kg;
- the probability that non-fatal health effects would occur from exposure to children's jewellery with a total lead concentration between 90 and 600 mg/kg and a cadmium concentration above 130 mg/kg; and
- the extent to which those non-fatal health effects would lead to other economic impacts.
Because information in these core areas is lacking and cannot feasibly be obtained, it was not possible to make a quantitative estimate of benefits from the proposal.
Qualitative benefits included avoidance of fatalities, illnesses and consequent medical costs, loss of lifetime earnings, and costs for special education, justice and corrections related to lead or cadmium poisoning. A break-even analysis suggested that benefits would outweigh costs if one fatality was avoided within approximately six years of adopting the proposed limits.
Benefits to industry
Removal of the migratable limit would simplify compliance testing for industry by eliminating any need to test for migratable lead. Currently, migratable testing is re-quired if total lead content is between 90 and 600 mg/kg. Total lead content tests are less expensive than migratable lead tests. Thus business (including small business) compliance costs may be reduced as a result of the proposed amendments. However, the size of this reduction is not directly measurable because under the current lead limits, migratable lead tests are required only if the total lead content is between 90 and 600 mg/kg, and the percentage of children's jewellery currently marketed in Canada that contains lead within this range is unknown.
Benefits to Government
Replacement of the current lead limits with a single total lead limit would also simplify testing for Health Canada and eliminate costs associated with migratable lead testing. The introduction of a specific cadmium limit would simplify compliance and enforcement activities relating to cadmium in children's jewellery and eliminate the need to assess samples on a product-by-product basis to determine if the cadmium content poses a danger to human health or safety.
“One-for-One” Rule
The “One-for-One” Rule does not apply, since the Regulations do not impose any administrative burden on industry.
Small business lens
The small business lens does not apply because the estimated nationwide cost impact is less than $1 million per year.
Consultation
A consultation document, the “Draft Proposal for Cadmium Guidelines in Children's Jewellery,” was posted on the Health Canada website from July to November 2011 for stakeholder comment. (see footnote 7) The document proposed 130 mg/kg total cadmium as a guideline limit for children's jewellery and requested industry feedback on this limit. Only one response was received, from an American industry association that called on Canada to adopt the cadmium requirements of the voluntary ASTM F2923-11, Standard Specification for Consumer Product Safety for Children's Jewelry.
An economic cost-benefit analysis of the proposed amendments was completed in March 2014. A survey questionnaire was distributed to 27 industry stakeholders, including one Canadian manufacturer, 12 wholesalers/retailers, 10 retailers, 2 industry associations, including an association representing small business, one regulatory agency in the United States, and a supplier of metal-testing equipment. Responses were received from 8 companies and one U.S.-based industry association.
Seven of the companies indicated that they did not expect any significant costs from the amendments. The remaining company indicated that it would carry significant compliance costs. Industry respondents in general expressed the view that regulatory requirements for children's jewellery should be harmonized with those of the United States.
Regulatory cooperation
Lead
Under the U.S. Consumer Product Safety Improvement Act (CPSIA), the lead limit for children's jewellery and other children's products is currently 100 mg/kg total lead, except for applied surface coatings on children's products, which have a 90 mg/kg total lead limit. The lead limits apply to all jewellery items intended for children aged 12 years or under.
The proposed amendments introduce requirements for affected products that are generally aligned with requirements in the United States. While the proposed amendments would result in slight differences between Canadian and U.S. requirements for lead, these differences are being maintained because they would either (i) ensure consistency for lead limits across the Canadian regulatory regime, (ii) be more protective of health, or (iii) ensure consistency with the common objective held by Health Canada and consumer product safety regimes in other jurisdictions to reduce the intentional use of lead to the greatest extent feasible.
Unlike the U.S. requirement, the CJR apply to all jewellery items intended primarily for children under 15 years of age. Health Canada chose the broader age limit because it is more reflective of industry marketing practices, which target the 10–14-year “tweens-young teens” age range as a single group.
While the proposed amendments would preserve a slight difference between Canadian and U.S. lead limits for certain products, they would ensure consistency for lead limits across the Canadian regulatory regime. There is currently a 90 mg/kg total lead limit under the CCPSA for all other regulated products that pose a similar lead exposure risk to children.
The proposed total lead limit of 90 mg/kg is only slightly different from the current U.S. limit of 100 mg/kg. A 10 mg/kg difference in lead limits would likely have no demonstrable impact on health. Either value (U.S. or Canadian limit) would preclude the intentional use of lead. In the past, industry has expressed concern that the different total lead limits for children's products in Canada and the United States have the potential to add unnecessary complexity and confusion for its members and to increase product testing costs. Industry has not provided any economic or other data as evidence of adverse impacts from the 10 mg/kg difference. Furthermore, there is no practical difference to industry for compliance testing purposes. A single total lead test would be sufficient to determine compliance with either limit.
Jewellery items that are compliant with the U.S. limit of 100 mg/kg total lead limit (but not the proposed 90 mg/kg limit) would not always result in recall action by Health Canada. Decisions are made on a case-by-case basis, taking into consideration various factors, such as past compliance history, type of product, availability on the market, previous amounts sold to consumers, and level of risk posed by the product.
The differences in lead content limits between this regulatory proposal and limits in other jurisdictions reflect the need to achieve internal regulatory consistency by aligning the lead limit for children's jewellery with the existing measures in place under the CCPSA to protect children against lead in other product categories that present a similar lead exposure risk.
Health Canada supports the Government of Canada's efforts to work towards greater alignment with American regulatory requirements in order to provide greater consistency for industry. The Department will continue to work with the U.S. CPSC and other international and industry partners to ensure children's products do not contain lead at levels that could harm children's health. However, Health Canada has decided not to align lead requirements for children's jewellery with a limit that is higher than Canada's existing limit for similar products.
Cadmium
The proposed addition of a 130 mg/kg total cadmium level to the CJR is not aligned with the U.S. measure, as there is no U.S. federal limit for cadmium in children's jewellery. Instead, a voluntary national standard, ASTM F2923-14, Standard Specification for Consumer Product Safety for Children's Jewelry, developed in 2011 and updated in 2014, is in place. The ASTM standard is applicable to jewellery items intended for children aged 12 years and under. It does not include any total cadmium limit, but requires migratable cadmium testing if total cadmium is greater than 300 mg/kg. The migratable cadmium limits vary depending on the material being tested and the size of the jewellery.
Health Canada does not consider the migratable cadmium limits in the ASTM standard to be sufficiently protective under the likely exposure situation in which an item becomes lodged in the digestive system over an extended period of time.
Compliance with the proposed limit of 130 mg/kg total cadmium content for children's jewellery in Canada would also mean compliance with the ASTM standard. As well, the 130 mg/kg limit is within the acceptable range currently in place in the EU. The EU currently imposes a total cadmium limit of 0.01% by weight (100 mg/kg ±50) for both adult and children's fashion jewellery.
Rationale
The lead or cadmium content of a product cannot be determined through visual inspection, and consumers and caregivers cannot effectively avoid exposure risks from children's jewellery items by their own actions. Regulatory intervention is needed to help manage the risk of lead and cadmium exposure. The results of stakeholder consultations indicate that costs to industry would be outweighed by the societal benefits of reduced lead and cadmium exposure to children.
The proposed amendments would protect children under the age of 14 years against adverse health risks posed by lead and cadmium in children's jewellery. However, the risks are most pronounced for children 4 years old or younger because of their normal mouthing behaviour. Children in this age group routinely place objects in their mouths and may repeatedly chew, suck or even accidentally swallow small items they find. Thus, children who are 4 years old and younger face a disproportionate level of risk and would be the age group likely to benefit the most from the current proposal.
The proposal would also protect the broader Canadian population against exposure to toxic metals, and reduce the amount of lead and cadmium in the human environment by discouraging its non-essential use in children's jewellery.
Specific total cadmium and lead limits would provide a clear compliance goal for industry and would give Health Canada the authority to take immediate enforcement action against any children's jewellery items that are not in compliance with the CJR.
Since 2011, Health Canada has had strengthened authority to request recalls of children's jewellery that exceeded the 130 mg/kg guideline limit on cadmium because the Department has the ability to invoke the CCPSA's GP provisions and orders regime in the event that a voluntary recall was not posted. Although this approach has been adopted for other issues and has successfully demonstrated a key aspect of the modern tools and authorities afforded by the CCPSA, invoking the provisions requires the extra burden of a risk assessment to support compliance actions. Establishing cadmium limits in regulations would allow Health Canada to take swifter action on non-compliant products and provides greater clarity for industry regarding acceptable limits.
Adoption of a 90 mg/kg total lead limit under the CJR would ensure a lower lead content in children's jewellery and promote internal alignment with lead content limits for other products regulated under the CCPSA. The 90 mg/kg total lead limit is considered health-protective and is consistent with the objective of reducing the potential for lead exposure as much as possible, since no “safe” level of lead exposure has been scientifically identified.
Implementation, enforcement and service standards
The proposed amendments would come into effect six months after the date of publication in the Canada Gazette, Part II. In the interim, Health Canada would continue to take corrective measures using the GP provisions and orders regime if children's jewellery containing high cadmium levels was found on the Canadian marketplace.
Compliance and enforcement of the proposed regulatory amendments would follow established departmental policy and procedures. Health Canada's cyclical enforcement (CE) approach for consumer products requires planned monitoring and enforcement surveys of all regulated products at regular intervals. The CE Program proactively monitors industry compliance with the CCPSA and its regulations through planned marketplace surveillance surveys within a recurring time frame. The frequency of CE surveys is based on the degree of risk and hazard associated with the regulated products.
Enforcement activities under the proposed amendments would be targeted towards products that pose the highest potential risk. Immediate and appropriate enforcement actions, such as removal from sale and recall of products already sold, would be taken against any non-compliant products.
A CE survey for children's jewellery may be carried out within six months of the coming into force of the amended CJR. The timing and scope of follow-up surveys would be determined by the results of this initial survey.
Contact
Sarah Sheffield
Project Officer
Risk Management Bureau
Product Safety Directorate
Healthy Environments and Consumer Safety Branch
Health Canada
123 Slater Street, 3504D
Ottawa, Ontario
K1A 0K9
Fax: 613-952-9138
Email: Sarah.Sheffield@hc-sc.gc.ca
PROPOSED REGULATORY TEXT
Notice is given that the Governor in Council, pursuant to section 37 of the Canada Consumer Product Safety Act (see footnote a), proposes to make the annexed Children's Jewellery Regulations.
Interested persons may make representations concerning the proposed Regulations within 75 days after the date of publication of this notice. All such representations must cite the Canada Gazette, Part I, and the date of publication of this notice, and be addressed to Sarah Sheffield, Project Officer, Risk Management Bureau, Consumer Product Safety Directorate, Healthy Environments and Consumer Safety Branch, Department of Health, Address Locator 4908B, 269 Laurier Avenue West, Ottawa, Ontario K1A 0K9 (fax: 613-952-2551; email: sarah.sheffield@hc-sc.gc.ca).
Ottawa, November 24, 2016
Jurica Čapkun
Assistant Clerk of the Privy Council
Children's Jewellery Regulations
Interpretation
Definitions
1 The following definitions apply in these Regulations.
children's jewellery means jewellery that is manufactured, sized, decorated, packaged, advertised or sold in a manner that appeals primarily to children under 15 years of age but does not include merit badges, medals for achievement or other similar objects normally worn only occasionally. (bijoux pour enfants)
good laboratory practices means practices that are in accordance with the principles set out in the Organisation for Economic Co-operation and Development document entitled OECD Principles of Good Laboratory Practice, Number 1 of the OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, ENV/MC/CHEM(98)17, the English version of which is dated January 21, 1998 and the French version of which is dated March 6, 1998. (bonnes pratiques de laboratoire)
Requirements
Lead content
2 Children's jewellery, when tested using good laboratory practices, must not contain more than 90 mg/kg of lead.
Cadmium content
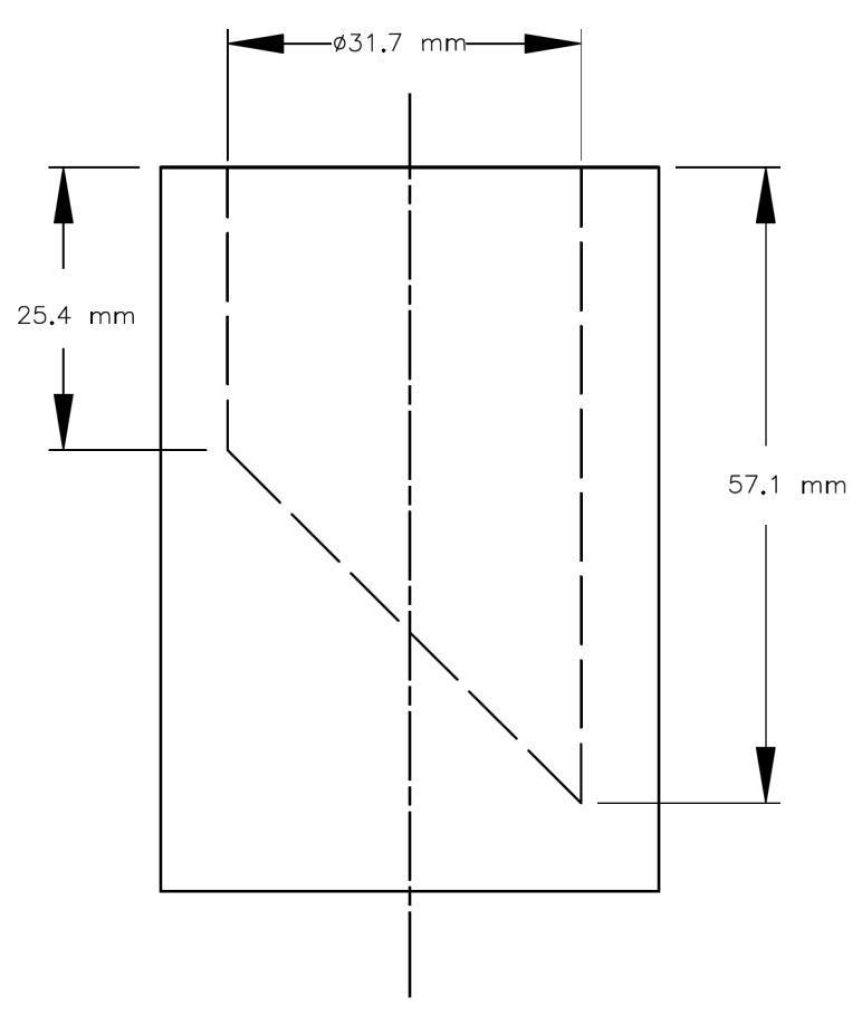
3 Children's jewellery, when tested using good laboratory practices, must not contain more than 130 mg/kg of cadmium if the jewellery item is small enough to be totally enclosed in the small parts cylinder illustrated in the schedule when a force of not more than 4.45 N is applied.
Repeal
4 The Children's Jewellery Regulations (see footnote 8) are repealed.
Coming into Force
Six months after publication
5 These Regulations come into force on the day that, in the sixth month after the month in which they are published in the Canada Gazette, Part II, has the same calendar number as the day on which they are published or, if that sixth month has no day with that number, the last day of that sixth month.
SCHEDULE
(Section 3)
Small Parts Cylinder
[49-1-o]