Vol. 151, No. 23 — November 15, 2017
Registration
SOR/2017-228 October 26, 2017
RADIATION EMITTING DEVICES ACT
Regulations Amending the Radiation Emitting Devices Regulations (Dental X-ray Equipment)
P.C. 2017-1291 October 26, 2017
Her Excellency the Governor General in Council, on the recommendation of the Minister of Health, pursuant to subsection 13(1) (see footnote a) of the Radiation Emitting Devices Act (see footnote b), makes the annexed Regulations Amending the Radiation Emitting Devices Regulations (Dental X-ray Equipment).
Regulations Amending the Radiation Emitting Devices Regulations (Dental X-ray Equipment)
Amendments
1 Section 2 of Schedule I to the Radiation Emitting Devices Regulations (see footnote 1) is replaced by the following:
2 Dental X-ray equipment, being radiation emitting devices that are designed primarily for the examination of dental and maxillofacial structures in living humans and that have an X-ray generating tube designed to be used outside the mouth.
2 Section 12 of Schedule I to the Regulations is replaced by the following:
12 Diagnostic X-ray equipment, being radiation emitting devices that use X-rays and are designed for the examination of the human body. The equipment does not include dental X-ray equipment, photofluorographic X-ray equipment, radiation therapy simulators and computer-assisted tomographic equipment.
3 Part II of Schedule II to the Regulations is replaced by the following:
PART II
Dental X-Ray Equipment
Interpretation
Definitions
1 The following definitions apply in this Part.
- aluminum means aluminum that has a degree of purity of 99.9% or higher and a density of 2.70 g/cm3. (aluminium)
- aluminum equivalent means the attenuation equivalent of an object, expressed in thickness of aluminum. (équivalent en aluminium)
- coefficient of variation means the ratio of the estimated standard deviation to the mean value of a series of measurements, determined by the formula

- where
- C is the coefficient of variation;
- S is the estimated standard deviation;
- X is the mean value of the measurements;
- Xi is the value of the ith measurement; and
- n is the number of measurements. (coefficient de variation)
- dental volumetric reconstruction means reconstruction of the three-dimensional attenuation distribution of all or part of the irradiated volume from a series of two-dimensional projections that are produced by an X-ray beam on an X-ray image receptor moving around the head of a patient. (reconstruction dentaire volumétrique)
- deviation, in respect of any parameter of dental X-ray equipment, means the percentage error between the measured value and the value indicated either on the equipment or in the accompanying information. (variation)
- digital X-ray image receptor means an X-ray image receptor that has a conversion method which is electrically powered. The conversion method may include single-step direct conversion or multi-step indirect conversion. (récepteur numérique d’image radiologique)
- effective image reception area means the part of the image reception area that is configured to receive an X-ray pattern that can be processed for display or storage. (surface réceptrice de l’image efficace)
- extra-oral, in relation to dental radiography, means that the X-ray image receptor is located outside the oral cavity. (extra-oral)
- hand-held, in respect of transportable dental X-ray equipment, means that the equipment, once it is placed into service, is held in or supported by the hand. (portatif)
- intra-oral, in relation to dental radiography, means that all or part of the X-ray image receptor is located inside the oral cavity. (intra-oral)
- irradiation time means the duration of an irradiation, measured as the interval between the instant when the air kerma rate rises for the first time to a value of 50% of the peak value and the instant when it drops for the last time below 50% of the peak value. (temps d’irradiation)
- lead equivalent means the attenuation equivalent of an object, expressed in thickness of lead. (équivalent en plomb)
- loading factor means a factor the value of which influences the X-ray tube load. It includes the following:
- (a) in the case of an X-ray beam that is produced by the discharge of the capacitor through an X-ray tube, the X-ray tube voltage and the amount of capacitor charge;
- (b) in the case of a field emission device in which the emission of electrons from the cathode is due solely to the action of an electric field, the X-ray tube voltage and the number of pulses; and
- (c) in any other case, the X-ray tube voltage and either
- (i) the X-ray tube current and irradiation time, or
- (ii) the current time product. (paramètre de charge)
- mode of operation means the technical state that is defined by a configuration of several predetermined loading factors and other settings for radiography or radioscopy, selectable simultaneously by the operation of a single control. (mode de fonctionnement)
- rectification type means the process by which the X-ray generator of dental X-ray equipment converts high voltage to X-ray tube voltage. (type de redressement)
- transportable, in relation to dental X-ray equipment, means that the equipment is constructed so that, once it is placed into service, it is capable of being moved from one place to another. (transportable)
- X-ray field means the area on a surface that is intersected by a radiation beam whose boundary is determined by the points where the air kerma drops to 25% of the air kerma at the centre of the area. (champ de rayonnement X)
- X-ray image receptor means a device that converts incident X-rays into a visible image or into a form that can be made into a visible image. (récepteur d’image radiologique)
Information and Labelling
Information
General requirements
2 The manufacturer, distributor and importer must ensure that all of the following information accompanies each piece of dental X-ray equipment:
- (a) the manufacturer’s name and civic address, and its postal address if different;
- (b) the model designation of the equipment;
- (c) the installation instructions;
- (d) any radiological safety procedures and additional precautions that are necessary because of any unique features of the equipment;
- (e) instructions for use that include
- (i) a description of the influence of the main settings or selections that are available to the operator on the radiation dose to the patient,
- (ii) if protection of the operator is affected by distance from the equipment, information on the impact of distance on the radiation dose, and
- (iii) all information necessary to minimize the operator’s exposure to radiation;
- (f) maintenance instructions;
- (g) procedures for quality control testing to be performed on the equipment, including how often the tests are to be performed and the acceptance criteria;
- (h) for each X-ray tube assembly,
- (i) the nominal focal spot sizes,
- (ii) the cooling curves for the anode and for the X-ray tube housing,
- (iii) the X-ray tube rating charts, and
- (iv) the focal spot position;
- (i) the duty cycles, its rectification type and its generator rating;
- (j) the nominal line voltage, the maximum line current and the line voltage regulation that are necessary to operate the equipment at the maximum line current;
- (k) the loading factors that constitute the maximum line current condition for the X-ray generator;
- (l) the recommended loading factors for each patient size;
- (m) when combinations of loading factors are indicated on the control panel by either a single reference to the combination or the value of only one of the loading factors that makes up the combination, the values of all the loading factors for each combination;
- (n) if the equipment can operate in automatic exposure control mode,
- (i) the accuracy limits of the automatic exposure control,
- (ii) the nominal shortest irradiation time in that mode, and
- (iii) the reproducibility of the air kerma relative to the range of loading factors, as the loading factors are adjusted by the automatic exposure control;
- (o) if the equipment can operate in a mode other than automatic exposure control mode, the operating range and the maximum deviation for any setting within the operating range for each loading factor;
- (p) if the equipment is battery-powered, the minimum state of charge that is necessary for it to operate;
- (q) if removable protective devices are specified for use with the equipment by the manufacturer, information about their effectiveness, application and use;
- (r) if dosimetric indications are displayed on the equipment, information and instructions on how to check and maintain their accuracy; and
- (s) for transportable equipment, recommendations for secure storage of the equipment against theft and unauthorized use.
Additional requirements — intra-oral equipment
3 In addition to the requirements set out in section 2, the manufacturer, distributor and importer must ensure that all of the following additional information accompanies each piece of intra-oral dental X-ray equipment:
- (a) the shape and dimensions of the exit field;
- (b) in the case of equipment that has a digital X-ray image receptor,
- (i) a description of the minimum performance criteria for the device that is used to display the images for diagnostic purposes,
- (ii) the nominal X-ray image receptor air kerma range that is needed for the intended use, and
- (iii) recommendations for typical loading factors at specified distances between the focal spot and the skin to achieve the air kerma referred to in subparagraph (ii);
- (c) the method by which the distance between the focal spot and the skin can be determined using the indicator specified in paragraph 7(f);
- (d) if the air kerma is indicated on the equipment, the maximum deviation;
- (e) if the air kerma is not indicated on the equipment,
- (i) the air kerma at a given distance from the focal spot for every selectable combination of loading factors, and
- (ii) the maximum deviation of the air kerma;
- (f) a method to calculate the dose area product using the air kerma and the exit field size; and
- (g) in the case of hand-held equipment,
- (i) values for the leakage radiation at the operator’s position and the method to assess the leakage radiation,
- (ii) guidance on how to avoid image degradation caused by motion of the X-ray source assembly during loading and the methods to assess the degradation, and
- (iii) the designation of a significant zone of occupancy, as follows:
- (A) dimensions of at least 60 cm x 60 cm with a height of at least 200 cm,
- (B) a drawing that indicates the boundaries of the zone in relation to clearly recognizable features of the equipment,
- (C) at least one profile of stray radiation in the zone with respect to the height above the floor — under representative operating conditions indicated — and that includes the point that receives the highest dose, and
- (D) a description of the testing methodology used to determine the profiles of stray radiation, including instructions for achieving the loading factors used in the testing if they are controlled only by an automatic control system.
Additional requirements — extra-oral equipment
4 In addition to the requirements set out in section 2, the manufacturer, distributor and importer must ensure that all of the following additional information accompanies each piece of extra-oral dental X-ray equipment:
- (a) a description of the geometric relationship between the focal spot, X-ray beam dimensions, patient position and image reception area;
- (b) if the air kerma is indicated on the equipment, the maximum deviation;
- (c) if the air kerma is not indicated on the equipment,
- (i) the air kerma at the entrance of the X-ray image receptor for every selectable combination of loading factors, and
- (ii) the maximum deviation of the air kerma;
- (d) the maximum deviation of the dose area product;
- (e) instructions on how to identify the location and dimensions of the effective image reception area;
- (f) for equipment in which any of the loading factors set out in items 1 to 3 and 5, column 1, of the table to subsection 30(1) vary during an irradiation, instructions on how to measure the deviation and on how to compare it with the maximum deviation set out in column 2 of that table; and
- (g) if a precalculated or measured current time product is indicated on the equipment, the lowest current time product or the combinations of loading factors that result in the lowest current time product.
Labelling
Presentation
5 The manufacturer, distributor and importer must ensure that all information that is required by this Part to be displayed on dental X-ray equipment is set out in a permanent, clear and legible manner on the specified surface of the equipment when it is fully assembled for use.
Function of controls
6 The manufacturer must ensure that all controls, warning lights and other indicators on the control panel are clearly labelled as to their function.
Displayed information
7 The manufacturer must ensure that all of the following information is displayed on dental X-ray equipment:
- (a) on the external surface of the control panel,
- (i) a statement that unauthorized use of the equipment is prohibited,
- (ii) a warning that hazardous X-rays are emitted when the equipment is in use, and
- (iii) one of the X-ray warning symbols set out in section 8;
- (b) on an external surface of the equipment,
- (i) the name of its manufacturer,
- (ii) its model designation,
- (iii) its serial number,
- (iv) the date of its manufacture, and
- (v) the country where it was manufactured;
- (c) on or near the external surface of the control panel — when combinations of loading factors are indicated on the control panel by either a single reference to the combination or the value of only one of the loading factors that makes up the combination — the values of all the loading factors for each combination;
- (d) on the external surface of the X-ray source assembly, with respect to the X-ray tube,
- (i) the name of its manufacturer,
- (ii) the model designation,
- (iii) its serial number, and
- (iv) the country of its manufacture;
- (e) on the external surface of the X-ray source assembly, the permanent filtration of the X-ray source assembly, expressed at a specified X-ray tube voltage either in millimetres of aluminum equivalent or as the thickness of any other material, together with its chemical symbol;
- (f) on the external surface of the X-ray source assembly, a mark that indicates the location along the X-ray beam axis of the focal spot on the anode target;
- (g) on the surface of any detachable beam-limiting device,
- (i) the name of its manufacturer,
- (ii) its model designation,
- (iii) its serial number,
- (iv) its quality equivalent filtration, if it is more than 0.2 mm aluminum equivalent, expressed at a specified X-ray tube voltage either in millimetres of aluminum equivalent or as the thickness of another material together with its chemical symbol, and
- (v) in the case of intra-oral equipment, its exit field size;
- (h) on the external surface of every fixed layer of material in the path of the X-ray beam incident on the patient — excluding any added filters and nonremovable materials in the X-ray tube assembly — the quality equivalent filtration, if it is more than 0.2 mm aluminum equivalent, expressed at a specified X-ray tube voltage either in millimetres of aluminum equivalent or as the thickness of another material together with its chemical symbols; and
- (i) on the external surface of the X-ray tube housing of hand-held intra-oral equipment, the words
- “WARNING: Hand-held operation increases operator radiation exposure due to proximity. See manufacturer safety information.”
- « ATTENTION : L’utilisation de l’appareil en mode portatif augmente l’exposition de l’opérateur au rayonnement en raison de la proximité. Consultez les renseignements de sécurité du fabricant. »
Warning symbol
8 The X-ray warning symbol must have the following characteristics:
- (a) it must be displayed in two contrasting colours;
- (b) it must be visible and identifiable from a distance of 1 m;
- (c) it must be at least 2 cm high and at least 2 cm wide;
- (d) it must bear the words “CAUTION: X-RAYS — ATTENTION : RAYONS X”; and
- (e) it must conform to one of the following:
- (i) the X-ray warning symbol:
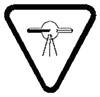
- (ii) the symbol ISO 361 in the report of the International Electrotechnical Commission entitled Graphical symbols for electrical equipment in medical practice, Publication IEC TR 60878: 2015, Third Edition, illustrated as follows:

- (iii) the symbol ISO 7010-W003 in the report of the International Electrotechnical Commission entitled Graphical symbols for electrical equipment in medical practice, Publication IEC TR 60878: 2015, Third Edition, illustrated as follows:

Construction and Functioning Standards
Control panel
9 The control panel of dental X-ray equipment must have all of the following features:
- (a) a visual indicator that warns the operator when one further actuation of a control will initiate the emission of X-rays;
- (b) a visual indicator that warns the operator when X-rays are being emitted and that is clearly visible during the emission;
- (c) if the equipment can emit X-rays while not in automatic exposure control mode, controls and visual indicators that enable the operator to select the loading factors or mode of operation before initiation of the irradiation;
- (d) if more than one X-ray source assembly is controlled by the same control panel, a visual indicator that shows, before initiation of the irradiation, which of the X-ray source assemblies is connected and ready to emit X-rays;
- (e) if the equipment allows for the selection of added filters by remote control or an automatic system, a visual indicator that shows which filter has been selected;
- (f) if the equipment is battery-powered, a visual indicator that shows whether the battery is adequately charged for the proper operation of the equipment;
- (g) for extra-oral equipment, an indicator that displays the dose area product; and
- (h) for extra-oral equipment, an indicator that displays the air kerma at the entrance of the X-ray image receptor, unless the air kerma is already provided in the accompanying information required by section 4.
Irradiation switch — functions
10 (1) Every irradiation switch of dental X-ray equipment must enable all of the following functions:
- (a) to initiate and to end an irradiation;
- (b) to permit the emission of X-rays only when the operator exerts continuous pressure on the switch;
- (c) to operate in a way that it is not possible to initiate an irradiation without first releasing the switch by which the previous irradiation was initiated; and
- (d) except in the case of hand-held intra-oral equipment, to permit the operator to stand at least 2 m from the X-ray source assembly when the X-ray tube is energized.
Hand-held intra-oral equipment
(2) Hand-held intra-oral equipment must also be equipped with a second irradiation switch, whether attached or remote, that gives the operator the option to stand at least 2 m from the X-ray source assembly when the equipment is supported on a stand.
Protected area
11 Dental X-ray equipment, except transportable equipment, must have a means to permit initiation of an irradiation by the operator from an area that is protected from radiation by structural shielding or distance.
Audible signal
12 Dental X-ray equipment must emit a signal, clearly audible to the operator, that indicates the end of an irradiation.
Line voltage fluctuations
13 Dental X-ray equipment must have a means appropriate to its rectification type to compensate for variations in X-ray tube voltage that are caused by line voltage fluctuations.
X-ray tube
14 The X-ray tube of dental X-ray equipment must be securely affixed to and aligned within the X-ray tube housing.
X-ray source assembly
15 The X-ray source assembly of dental X-ray equipment must maintain its required position or trajectory without drift or vibration during operation.
Multiple X-ray source assemblies
16 If more than one X-ray source assembly of dental X-ray equipment is controlled by the same control panel, the equipment must have a visual indicator on or near each X-ray tube housing that shows which of the X-ray source assemblies has been selected.
Filtration
17 (1) Dental X-ray equipment must have radiation-absorbing filters that meet the following requirements:
- (a) the filters must be securely affixed to the exit port of the X-ray tube housing or to the beam-limiting device, or to both; and
- (b) the filters must provide the following degree of attenuation of the X-ray beam:
- (i) for each X-ray tube voltage peak value set out in column 1 of the table to this subsection, a first half-value layer of aluminum that is not less than the value set out in column 2, and
- (ii) in any other case, a first half-value layer of aluminum that is not less than the value obtained by linear interpolation or extrapolation from that table.
TABLE
| Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
60 |
2.2 |
2 |
70 |
2.5 |
3 |
80 |
2.9 |
4 |
90 |
3.2 |
5 |
100 |
3.6 |
6 |
110 |
3.9 |
7 |
120 |
4.3 |
8 |
130 |
4.7 |
9 |
140 |
5.0 |
10 |
150 |
5.4 |
Total filtration — intra-oral equipment
(2) Despite paragraph (1)(b), if intra-oral dental X-ray equipment operates at a maximum nominal X-ray tube voltage of 70 kV or less, it may have a total filtration of at least 1.5 mm aluminum equivalent.
Automatic exposure control
18 Dental X-ray equipment that has an automatic exposure control must have the following features:
- (a) a means to automatically end the irradiation when,
- (i) in the case of extra-oral equipment, either
- (A) the product of the X-ray tube voltage, X-ray tube current and irradiation time exceeds 64 kJ per irradiation, or
- (B) the current time product exceeds 640 mAs per irradiation, and
- (ii) in the case of intra-oral equipment, either
- (A) the product of the X-ray tube voltage, X-ray tube current and irradiation time exceeds 3.2 kJ per irradiation, or
- (B) the current time product exceeds 32 mAs per irradiation;
- (b) a visual indicator or audible signal to warn the operator that the irradiation has ended because one of the limits set out in paragraph (a) has been reached; and
- (c) a reset control that must be activated manually once one of the limits set out in paragraph (a) has been reached and before another irradiation under automatic exposure control can be initiated.
Minimum distance — focal spot to skin
19 The minimum distance between the focal spot and the skin must be
- (a) 15 cm, in the case of extra-oral equipment; and
- (b) 20 cm, in the case of intra-oral equipment.
Maximum deviation — focal spot to image receptor
20 If the distance between the focal spot and the X-ray image receptor of extra-oral dental X-ray equipment is adjustable, the equipment must have a visual indicator that shows the distance that is selected and that has a maximum deviation of 5%.
Beam-limiting device — extra-oral equipment
21 (1) Extra-oral dental X-ray equipment must have a beam-limiting device that meets the following requirements:
- (a) when used for dental volumetric reconstruction with a circular image reception area, the device must operate so that
- (i) the X-ray field does not exceed the boundary of the effective image reception area by more than 2 cm, measured along any diameter, and
- (ii) at least 90% of the area of the X-ray field overlaps the effective image reception area;
- (b) when used for dental volumetric reconstruction with a rectangular image reception area, the device must operate so that
- (i) along each of the two axes of the image reception area, the edges of the X-ray field do not exceed the corresponding edges of the effective image reception area by more than 2 cm or 3% of the indicated distance between the focal spot and the image receptor, whichever is larger, when the image reception plane is perpendicular to the X-ray beam axis, and
- (ii) the sum of the discrepancies on both axes does not exceed 3 cm or 4% of the indicated distance between the focal spot and the X-ray image receptor, whichever is larger;
- (c) when used for projection radiography, the device must operate so that the X-ray field does not exceed the boundary of the effective image reception area; and
- (d) when used for narrow beam scanning radiography, the device must operate so that
- (i) along the axis of the image reception area that is parallel to the direction of the scanning, the X-ray field does not exceed the boundary of the effective image reception area by more than 1 mm on each side, and
- (ii) along the axis of the image reception area that is perpendicular to the direction of the scanning, the X-ray field does not exceed boundary of the effective image reception area.
Beam-limiting device — intra-oral equipment
(2) Intra-oral dental X-ray equipment must have a beam-limiting device that meets the following requirements:
- (a) the device limits the X-ray field to a circle of not more than 6 cm diameter, measured at the point at which the beam exits the equipment;
- (b) if the device has an optional means to limit the exit field to a rectangular shape, it must operate so that the rectangle fits inside that circle;
- (c) if the device has a rectangular exit field, it must operate so that the exit field can rotate with respect to the X-ray beam axis; and
- (d) in the case of equipment that has an integrated direct digital X-ray image receptor, the device has a means to limit the X-ray field to a rectangular shape that does not exceed the boundary of the effective image reception area by more than 1 cm in the diagonal.
Incremental settings
22 When irradiation times are 0.08 s or greater, the range of settings for the X-ray tube current, irradiation time or current time product of dental X-ray equipment must be in increments that are not greater than the decimal multiples and submultiples of 1.00, 1.25, 1.60, 2.00, 2.50, 3.20, 4.00, 5.00, 6.30 and 8.00.
Modes of operation
23 Dental X-ray equipment that has more than one mode of operation must be constructed in accordance with the following requirements:
- (a) in the case of extra-oral equipment that does not have an integrated direct digital X-ray image receptor, the step size of the adjustment between adjacent settings of the current time product must not be greater than 1.6 mAs; and
- (b) in the case of intra-oral equipment,
- (i) the step size of the adjustment between adjacent settings of the current time product must not be greater than 1.6 mAs, and
- (ii) the ratio of the maximum to minimum current time product settings must be at least 4:1.
Current time product
24 For each selectable X-ray tube voltage of intra-oral dental X-ray equipment, the ratio of the maximum to minimum current time product settings must be at least 16:1.
Primary shielding
25 Extra-oral dental X-ray equipment must have primary protective shielding that completely overlaps the X-ray field and that provides the following minimum attenuation:
- (a) 0.5 mm lead equivalent, in the case of a nominal X-ray tube voltage of 90 kV or less; and
- (b) 2 mm lead equivalent, in the case of a nominal X-ray tube voltage of more than 90 kV.
Attenuation equivalent
26 All parts of extra-oral dental X-ray equipment that are located in the path of the X-ray beam between the patient and the X-ray image receptor must, when taken together, have an attenuation of not more than 1.2 mm aluminum equivalent, as determined at the highest X-ray tube voltage of the equipment.
Hand-held intra-oral equipment
27 Hand-held intra-oral dental X-ray equipment must have all of the following features:
- (a) a permanently affixed backscatter shield that has an attenuation of at least 0.25 mm lead equivalent, as determined at a nominal X-ray tube voltage of 70 kV;
- (b) a means to prevent unauthorized operation; and
- (c) a means to allow the equipment to be supported in a way that it maintains its position so that it is not necessary for the operator to hold the equipment during operation.
X-ray tube voltage
28 The minimum nominal X-ray tube voltage of dental X-ray equipment must be at least 60 kV.
Functioning requirements
29 Dental X-ray equipment must function in accordance with sections 30 to 35 under the following conditions:
- (a) the unloaded line voltage must not vary by more than 1% of its nominal value; and
- (b) the line voltage must be regulated so that it does not vary by more than 6% when the line is fully loaded at the maximum rated line current of the equipment.
Loading factors
30 (1) Subject to subsection (2), for any combination of loading factors, the measured value of a loading factor set out in column 1 of the table to this subsection must not deviate from the indicated value by more than the amount set out in column 2.
TABLE
| Item |
Column 1 |
Column 2 |
|---|---|---|
1 |
X-ray tube voltage |
10% |
2 |
X-ray tube current |
20% |
3 |
Irradiation time for extra-oral dental X-ray equipment |
5% + 50 ms |
4 |
Irradiation time for intra-oral dental X-ray equipment |
5% or 20 ms, whichever is greater |
5 |
Current time product for extra-oral dental X-ray equipment |
10% + 0.2 mAs |
Exception — intra-oral equipment
(2) In the case of intra-oral dental X-ray equipment that has a one-peak high-voltage generator, the maximum deviation set out in item 4, column 2, of the table to subsection (1) does not apply when the irradiation time is shorter than 0.1 s.
Coefficient of variation — air kerma
31 (1) For any combination of loading factors of dental X-ray equipment, the coefficient of variation of any five consecutive air kerma measurements — taken at the same point along the X-ray beam axis within a one-hour period — must be 0.05 or less.
Internally powered equipment
(2) Dental X-ray equipment that is internally powered must meet the requirement set out in subsection (1) over the whole range of usable charging levels of the internal supply.
Maximum deviation — air kerma
32 The maximum deviation of the air kerma for dental X-ray equipment must not exceed 50%.
Maximum deviation — dose area product
33 The maximum deviation of the dose area product for extra-oral dental X-ray equipment must not exceed 50%.
Air kerma — linearity
34 (1) Subject to subsection (2), for any selected value of X-ray tube voltage and over the whole range of current time product settings, for any two settings of the current time product that do not differ by more than a factor of 2, the quotients of the average of the air kerma measurements divided by the corresponding indicated current time product must not differ by more than 0.10 times their sum, as determined by the following formula, where X is the quotient of the average of the air kerma measurements divided by the indicated current time product, calculated at each of the two settings of the current time product:
|X1 – X2| ≤ 0.1(X1 + X2)
Exception — intra-oral equipment
(2) In the case of intra-oral dental X-ray equipment that has a one-peak high-voltage generator, the shortest irradiation time in the range of current time product settings is 80 ms.
Leakage radiation — loading state
35 (1) Leakage radiation from the X-ray source assembly of dental X-ray equipment must not exceed the following air kerma rate when the equipment is operated at the nominal X-ray tube conditions of loading that correspond to the maximum specified energy input in one hour:
- (a) in the case of extra-oral equipment, 1.0 mGy/h; and
- (b) in the case of intra-oral equipment,
- (i) 1.5 µGy/h for hand-held intra-oral equipment, and
- (ii) 0.25 mGy/h for all other intra-oral equipment.
Detection area
(2) For the purpose of subsection (1), the air kerma rate must be averaged over a detection area of 100 cm2 — of which no linear dimension is greater than 20 cm — that is 1 m from the focal spot.
Leakage radiation — extra-oral equipment in non-loading state
(3) In the case of extra-oral dental X-ray equipment that is not in the loading state, the leakage radiation from the X-ray source assembly must not exceed an air kerma rate of 20 µGy/h.
Detection area
(4) For the purpose of subsection (3), the air kerma rate must be averaged over a detection area of 10 cm2 — of which no linear dimension is greater than 5 cm — that is 5 cm from any accessible surface of the equipment.
Transitional Provision
4 The Radiation Emitting Devices Regulations, as they read immediately before the date of the coming into force of these Regulations, continue to apply in respect of dental X-ray equipment that was manufactured before the day on which these Regulations come into force.
Coming into Force
5 These Regulations come into force on the day that, in the sixth month after the month in which they are published in the Canada Gazette, Part II, has the same calendar number as the day on which they are published or, if that sixth month has no day with that number, the last day of that sixth month.
REGULATORY IMPACT ANALYSIS STATEMENT
(This statement is not part of the Regulations.)
Issues
Dental radiography is one of the most valuable tools in the field of dental care, supporting the diagnosis, treatment and management of dental health concerns. It is estimated that nearly 14 million Canadians receive dental X-rays each year, a process that involves exposure to ionizing radiation. Since this type of radiation is capable of damaging the deoxyribonucleic acid (DNA) of healthy cells, which may potentially lead to cancer, the radiation safety of dental X-ray equipment imported, sold and leased in Canada must be carefully managed.
The widely adopted model for protection from ionizing radiation risk assumes that the probability of cancer from radiation exposure is proportional to the dose received. As such, the risk of harm from dental X-rays is generally very low for individuals — given that the radiation dose is low for each exposure — relative to other types of medical imaging examinations. From a public health standpoint, however, the risk is more significant due to the large number of Canadians receiving dental X-rays. To help protect the Canadian population as a whole, dental X-ray equipment imported, sold and leased in Canada must meet requirements of the Radiation Emitting Devices Act (REDA) and the Radiation Emitting Devices Regulations.
The Radiation Emitting Devices Regulations (the Regulations) are no longer aligned with the current international standards of the International Electrotechnical Commission (IEC), updated in 2012. Amendments to the Regulations in order to align with these standards are necessary to strengthen Canada’s regulatory system and
- (1) set out strengthened radiation safety requirements for new and modern dental X-ray equipment (e.g. a requirement to increase the minimum peak X-ray tube voltage from 50 kV to 60 kV reduces unnecessary radiation exposure of patients to low energy X-rays that do not contribute to image formation);
- (2) address a broader scope of dental X-ray technologies (e.g. cone beam computed tomography [CBCT] and hand-held equipment); and
- (3) require manufacturers to provide more information to support optimized equipment use (e.g. quality control specifications).
The current standards of the IEC have already been recognized throughout the European Union and by the United States.
Strengthen radiation safety requirements
Ionizing radiation, such as X-rays, can result in deleterious health effects. Canada’s regulations will be more effective in protecting operators and patients of dental X-ray equipment if they reflect the latest scientific information related to radiation safety requirements. The current Regulations are no longer aligned with the current IEC Standards, which are a benchmark for industry. This represents a gap within Canada’s current regulatory framework and could put both operators and patients at risk of receiving unnecessary doses of radiation during the operation of dental X-ray equipment.
Address a broader scope of dental X-ray technologies
Advancements in dental X-ray technologies have resulted in additional types of dental X-ray equipment coming into Canada (e.g. CBCT). The Regulations do not specifically address the current breadth of technology available to dental professionals. Without proper regulatory oversight of new technologies and technological advancements in existing technologies, operators and patients could be exposed unnecessarily to higher than needed doses of radiation.
In addition, transportable hand-held dental X-ray equipment is not available for use in Canada, as it is currently not compliant with the radiation safety requirements of the Regulations. However, transportable hand-held dental X-ray equipment would provide dental practitioners access to another diagnostic tool, for use in situations where traditional wall- or stand-mounted dental X-ray equipment is not feasible (i.e. emergency medicine). These regulatory amendments address new technologies such as dental volumetric reconstruction technologies (e.g. CBCT) and transportable hand-held X-ray equipment.
Provide more information to support optimized equipment use
Optimization involves using the lowest dose of radiation necessary for a given clinical purpose (e.g. to produce an X-ray image that is of adequate diagnostic quality). The current Regulations require information necessary for the safe and proper operation of the equipment but do not have specific requirements for information on optimization. To address this issue, these amendments require the manufacturer, importer, or distributor of dental X-ray equipment to provide additional information on equipment design, available settings and their impact on radiation output, instructions on their use and quality control. This will provide operators with the means to optimize equipment use, thereby protecting both themselves and patients from unnecessary radiation exposure.
Background
In Canada, radiation protection in dentistry is a shared responsibility between the federal, provincial and territorial governments as well as the dental professionals involved in the delivery of dental care. Health Canada administers legislation applicable to the importation and sale of dental X-ray equipment under the REDA and the Regulations, as well as the Food and Drugs Act and the Medical Devices Regulations. Provincial and territorial governments and professional dental associations have made rules pertaining to the installation and safe use of this equipment.
The Consumer and Clinical Radiation Protection Bureau (CCRPB) within Health Canada administers the REDA and its Regulations in order to help protect the health of Canadians from potential hazards associated with radiation emitting devices, including dental X-ray equipment. The REDA includes provisions respecting the sale (including re-sale), lease and importation of radiation emitting devices, and authorizes the making of regulations pertaining to, among other things, the labelling, packaging, advertising, construction and functionality of radiation emitting devices.
Schedule II, Part II (Dental X-ray Equipment with an Extra-oral Source) of the Regulations, prescribes particular radiation safety standards for dental X-ray equipment, and was last amended on May 5, 1993.
Currently, the Regulations provide general regulatory oversight to wall-mounted, intra-oral equipment, cephalometric and panoramic dental X-ray equipment, but they do not adequately address the technological advancements that have occurred with this equipment. They permit the importation of CBCT dental X-ray equipment but prohibit the importation and sale of transportable hand-held dental X-ray equipment. The latter does not meet current safety requirements in the Regulations that require the irradiation switch to allow the operator to stand at least 3 m from the device.
More sophisticated types of wall-mounted, cephalometric and panoramic dental X-ray equipment are coming to Canada and although CBCT equipment is permitted under the current Regulations, there is nothing specifically that addresses the innovations respecting this technology within the Regulations. Aligning the Regulations with aspects of the 2012 IEC Standards will enhance Canada’s radiation safety requirements for these types of equipment, thereby providing greater protection to patients and operators. These amendments also permit the importation and sale of transportable hand-held dental X-ray equipment, with provisions for safety requirements specific to hand-held devices.
Objectives
The objectives of the regulatory amendments are to strengthen radiation safety requirements, in alignment with IEC Standards, for new equipment (i.e. equipment manufactured after the coming-into-force date of the amended Regulations), modern equipment (i.e. more sophisticated types of equipment currently permitted but not adequately addressed under the existing Regulations), and new technology (i.e. transportable hand-held equipment that is currently prohibited under the existing Regulations); broaden the scope of the Regulations to address new and modern dental X-ray technologies; and require the provision of information to support optimization of equipment use.
There is a need to update the regulatory requirements for dental X-ray equipment in order to enable Health Canada to exercise appropriate regulatory oversight on the sale, resale, lease, and importation of dental X-ray equipment in Canada to ultimately enhance the protection of operators and patients from the radiation emitted by those devices.
Description
The Regulations set out requirements respecting information, labelling, construction, and functioning of radiation emitting devices.
The amendments update such requirements for dental X-ray equipment, including conventional wall-mounted and mobile dental X-ray equipment, panoramic equipment, cephalometric equipment, CBCT equipment and transportable hand-held dental X-ray equipment, in the following ways:
Update information and labelling requirements for dental X-ray equipment
The amendments require manufacturers to provide additional information for each piece of dental X-ray equipment, including quality control procedures and dosimetric information. These information requirements are more extensive than what is currently addressed in the Regulations and provide operators with the ability to properly operate and maintain their machines, ensuring consistent and appropriate patient dose delivery.
These amendments also introduce a new requirement for a label on transportable hand-held dental X-ray equipment. The label will inform operators of radiation safety considerations associated with transportable hand-held equipment and be intended to promote safe use and mitigate radiation risks to operators.
Update construction standards
Requirements in the Regulations are amended with respect to such features as minimum tube voltage setting (increasing from 50 kV to 60 kV), exit field size for intra-oral devices (reducing the maximum diameter from 7 cm to 6 cm), radiation absorbing filters (increasing the minimum attenuation requirements for extra-oral devices), X-ray field limitations (e.g. requirements specific to CBCT machines) and focal spot to skin distance (increased to 20 cm for intra-oral devices). These changes will reduce the radiation risk to which the patient and operator are exposed during operation of the X-ray device.
In addition, these amendments enable the importation and sale of transportable hand-held intra-oral dental devices in Canada while introducing requirements to mitigate radiation risks from these devices.
Update functioning standards
Functioning standards are criteria that require equipment to perform within certain specified parameters under normal conditions of use. The regulatory amendments update functioning standards, including requirements for improved accuracy of loading factors, and a more restrictive limit on leakage radiation, which will help to protect both operators and patients from receiving unnecessary doses of radiation from dental X-ray equipment.
“One-for-One” Rule
The new standards set out requirements pertaining to labelling, construction, functioning, and information to accompany the device for its safe and proper operation, but do not introduce any activities that are considered to be an administrative burden to importers and sellers. There is also limited manufacturing of these devices in Canada. Therefore, the “One-for-One” Rule does not apply to these Regulations.
Small business lens
The small business lens does not apply because the nationwide cost impacts of these amendments are less than $1 million annually. The majority of manufacturers of dental X-ray equipment have already aligned their construction and function specifications with international standards (i.e. IEC Standards). Therefore, any cost associated with these amendments is expected to be minor.
Consultation
Health Canada conducted a targeted stakeholder consultation from August to November 2013 to solicit early input from stakeholders on potential amendments to the Regulations. Comments were received from a total of 13 separate stakeholders, including representation from provincial government radiation authorities, dental industry, dental professionals associations, federal government departments, academia and a private radiation safety company.
Health Canada’s approach to align its Regulations with international equipment standards (i.e. IEC Standards) received strong support from the stakeholders. Some stakeholders (including provincial government radiation authorities) sought guidance on CBCT, such as quality control requirements and training for operators. To address this, the amendments require manufacturers, distributors and importers to provide quality control information on all dental X-ray equipment for sale and lease in Canada, including dental volumetric reconstruction equipment.
Transportable hand-held dental X-ray equipment was identified by some stakeholders as a tool that could potentially provide additional benefits to Canadians in certain situations, with respect to patient accessibility, when compared to transportable mobile dental X-ray equipment that is currently authorized for sale and import in Canada. However, support was tempered by the stipulation that they only be used when the use of a fixed or mobile unit was not feasible (e.g. transportable hand-held devices should not be used in standard dental offices). The amendments permit the importation, sale and lease of transportable hand-held dental X-ray equipment in Canada and set out requirements to mitigate risks from these devices such as the inclusion of a second irradiation switch that gives the operator the option to be at least 2 m from the X-ray source assembly when the equipment is supported on a stand.
Prepublication
The proposed regulatory amendments were prepublished in the Canada Gazette, Part I, on June 18, 2016, for a 75-day comment period. Health Canada received comments from 14 stakeholders during the consultation period, including manufacturers of hand-held X-ray equipment, national dental professional associations and radiology groups, medical physicists, university dental departments, and professionals with clinical practices, including in hospitals and medical centres.
The majority of comments received were supportive of the proposed regulatory amendments, in particular, the alignment with IEC Standards and the provisions which would enable the introduction of hand-held X-ray equipment in Canada. Advantages and benefits cited for hand-held equipment included use in forensic applications (although this is outside of the scope of the proposed amendments), use in critical care settings, use for special needs and medically compromised patients, and patients unable to easily access dental care, such as those in remote communities or long-term care facilities.
Specific feedback was provided on some of the safety measures introduced to mitigate the potential risks of hand-held equipment. These comments included the possible burden of providing a stand, concerns of a tripping hazard with the secondary irradiation switch, and the practicality of a log-in code as a means to prevent unauthorized operation.
One industry stakeholder felt that the requirement to enable hand-held dental X-ray equipment to be mounted to a stand would require a redesign of their current devices and would impose a financial burden. Health Canada’s analysis of the hand-held dental X-ray equipment industry shows that a number of manufacturers already enable their devices to be mounted onto stands, which can accompany the units being sold. In addition, the intent of the regulatory amendment is not that the manufacturer must provide the stand, but rather that their device must include “a means to allow” the device to be put on a stand or otherwise securely supported. This requirement to provide users with the option to mount the hand-held device on a stand (i.e. not hold it in their hand) is necessary for the safety of the operator in order to minimize occupational radiation exposure in alignment with the ALARA (as low as reasonably achievable) concept.
With respect to the secondary irradiation switch, some stakeholders were concerned that it could pose a tripping hazard for operators. Since the provision is not intended to imply that the secondary irradiation switch be permanently attached or necessarily involve a cord, it has been reworded for greater clarity. This requirement provides users with the option to use distance as a method to reduce their radiation exposure in alignment with the concept of ALARA.
A few stakeholders stated that they did not support the requirement for a log-in code in order to operate the hand-held dental X-ray device, since they believed it would not be practical. However, the regulatory amendments do not specify the type of security measure to be used — only that a means to prevent unauthorized operation is required for safety purposes. Furthermore, some hand-held X-ray devices already have log-in code capabilities. As a result, the regulatory provision has not changed.
Some stakeholders provided comments regarding the use of hand-held devices (e.g. qualifications of personnel using the equipment, level of training), which is not within the scope of the Regulations. Provincial/territorial governments and professional dental associations may establish rules pertaining to the installation and safe use of dental X-ray equipment, whereas the Regulations set out information, construction, and functioning requirements for dental X-ray equipment. The regulatory amendments require manufacturers to include necessary information to enable the safe and proper usage of hand-held dental X-ray equipment. Guidance on use of hand-held devices will be addressed in a planned update of Safety Code 30: Radiation Protection in Dentistry. Health Canada safety codes may be referenced and used as guidance by other government groups (including provinces and territories) and stakeholders when establishing requirements for use (e.g. training, handling, maintenance, etc.).
Finally, some stakeholders sought clarification regarding certain regulatory requirements. In response Health Canada will develop a guidance document concerning the Regulations.
The following clarification changes have also been made to the amendments (the sections referenced below correspond to those found in the proposed regulatory amendments published in the Canada Gazette, Part I):
- Under Information and Labelling, paragraph 2(r), which stated, “if the equipment does not include an integrated X-ray image receptor, performance criteria for X-ray image receptors that are compatible with the equipment;” was removed, as it was determined to be burdensome with no safety benefit;
- The phrase “for facility shielding” was removed from paragraph 2(t), as it was considered burdensome and would be more appropriately addressed in updates to Health Canada Safety Code 30;
- Section 10, Irradiation Switch, was revised to clarify that the secondary irradiation switch is not required to be permanently attached to the device; and
- Sections 19, 21(2)(a) and 22 were revised for overall readability.
Rationale
These amendments update Canada’s regulatory oversight for radiation safety on new and modern dental X-ray equipment technologies and align Canada’s regulations with the current IEC Standards, which are already employed by the United States and the European Union. This will help strengthen radiation safety for both patients and operators by requiring that all dental X-ray equipment imported and sold in Canada support optimized radiation doses to patients and reduced exposures to operators. To achieve this, the amendments require manufacturers, importers and distributors to meet equipment requirements for information, labelling, construction and performance.
As the quality and construction of dental X-ray equipment may vary across manufacturers, some of these differences may put operators and patients at increased risk for higher received doses. Updating the regulatory requirements so that devices imported and sold in Canada meet internationally established standards for safety and performance helps ensure optimized radiation doses to patients and operators.
To illustrate how these amendments help optimize radiation doses to patients and operators, the amendments limit the X-ray field for intra-oral devices from the current limit of 7 cm diameter to no more than 6 cm, thus reducing the dose area product by about 25%. The dose area product is a measure that takes into account both radiation dose and how much of the body is receiving the radiation, and therefore is relative to radiation risk. These amendments also require accompanying documents to include a method to determine the dose area product from the device, as well as quality control information, both of which will help the operator to ensure the settings on the dental X-ray device are producing an appropriate dose area product on an ongoing basis. Ultimately, this will help operators protect themselves and their patients from exposure to unnecessary doses of radiation.
These amendments also provide operators with the opportunity to have access to hand-held dental X-ray equipment which may offer benefits in specific situations where the use of traditional wall- or stand-mounted devices is not feasible (i.e. emergency medicine).
In addition to increasing radiation safety requirements, these amendments will offer economic benefits to Canada. Due to discrepancies that exist between the radiation safety requirements of the current Canadian dental X-ray regulations and the latest IEC Standards, manufacturers and importers of certain types of dental X-ray equipment (e.g. transportable hand-held dental X-ray devices) are currently not importing or selling these devices in Canada, as modifications would be required in order to comply with the Canadian regulations. As nearly all dental X-ray equipment is manufactured outside of Canada and is designed to meet IEC Standards, the required modifications to import or sell certain devices in Canada currently result in a technical barrier and create additional costs which may be passed on to the consumer. By aligning with the current IEC Standards, these amendments will reduce the technical barrier to manufacturers, importers, and distributors that do not currently sell or import products in Canada because they do not wish to modify their products.
In terms of the potential costs to business (industry) as a result of the regulatory proposal, it is estimated that compliance costs for implementing the proposed amendments are minimal. Based on Innovation, Science and Economic Development Canada trade data from 2013, approximately 2 000 dental X-ray devices are imported into Canada per year. Given that the majority of dental X-ray devices are compliant with IEC Standards, it is estimated that no greater than 25% of those devices would be non-compliant with the proposed amendments. The costs associated with bringing a device into compliance, which include generation of new content, translations, printing of manuals, etc., are approximately $60; therefore, it is roughly estimated that the compliance costs for implementing the proposed amendments will be approximately $30,000 Canadian dollars per year. As most dental X-ray equipment is already manufactured to meet the current IEC Standards, the majority of the costs associated with these amendments are expected to relate to updating the information contained in manuals and labels, as well as some minor modifications to the construction of the devices.
Implementation, enforcement and service standards
In order to assist the dental industry transition, the amendments will come into force six months after the day that they are published in the Canada Gazette, Part II, allowing manufacturers sufficient time to align themselves with the amendments.
These amendments do not alter existing compliance mechanisms. Compliance and enforcement will continue to be undertaken by Health Canada inspectors under the authority of the REDA and the Regulations.
The current Regulations will continue to apply to dental X-ray equipment that was manufactured before the day on which these amendments come into force.
Additional guidance for industry (e.g. guidance document for industry, Qs and As document, or notice on Health Canada website) will be developed by Health Canada to assist with interpretation of the Regulations. Furthermore, a planned update of Safety Code 30: Radiation Protection in Dentistry will include guidance on use of the broader scope of dental technologies addressed in the Regulations (e.g. cone beam computed tomography [CBCT] and hand-held equipment). Updates and additional guidance will be communicated through various means, including the Federal-Provincial-Territorial Radiation Protection Committee (FPT-RPC).
Contact
Tara Bower
Director
Office of Science Policy, Liaison and Coordination
Environmental and Radiation Health Sciences Directorate
Healthy Environments and Consumer Safety Branch
Health Canada
Address Locator 4908D
269 Laurier Avenue West
Ottawa, Ontario
K1A 0K9
Fax: 613-952-5397
Email: ccrpb-pcrpcc@hc-sc.gc.ca
- Footnote a
S.C. 2016, c. 9, s. 28 - Footnote b
R.S., c. R-1 - Footnote 1
C.R.C., c. 1370